Abstract
Introduction : Optimal post-remission therapy for older adults with AML is unclear. Lenalidomide (LEN) is an oral immunomodulatory agent that reconstitutes T- and NK-cell immunodeficiency in patients with other malignancies. These immunomodulatory properties could benefit patients with Acute Myeloid Leukemia (AML) in remission. LEN, alone and in combination, has led to complete remissions in older patients with AML treated with doses above those approved for other malignancies. We sought the optimal dose of LEN in older AML patients who are not candidates for intensive post-remission therapy.
Methods: Subjects were: age ≥ 60 with AML; had 1-2 prior courses of induction chemotherapy; in CR, CRi, PR or had residual AML with ≤ 1 x 109/L circulating blasts; poor candidates for cytotoxic consolidation therapy as deemed by their physician or patient preference. A Bayesian dose-finding design was used to assign subjects to 3 cohorts (25 mg, 35 mg and 50 mg daily days 1-28 for one cycle). Subjects without progressive AML after cycle 1 then received up to 12 months of LEN 10 mg daily. Dose-limiting toxicities (DLTs) were CTCAEv4 grade ≥3 non-hematologic toxicity during cycle 1 and grade ≥3 hematologic toxicity beyond day 35 of cycle 1. AML response and relapse were measured by International Working Group criteria. Geriatric assessments were performed before and after cycle 1. Measures of T- and NK-cell immunity performed before and after months 1 and 4 included flow cytometry to quantify circulating T- and NK-cell subsets as well as NK-cell cytotoxicity using subject peripheral blood mononuclear cells as effector cells and K562 cell lines as targets. Effector cell-to-K562 ratios (E:T) were titrated between 1:100 and 1:1, and the percentage of dead K562 cells was plotted against E:T ratio. Cytotoxicity was reported as the slope of the curve of the percentage of dead K562 cells versus E:T ratio.
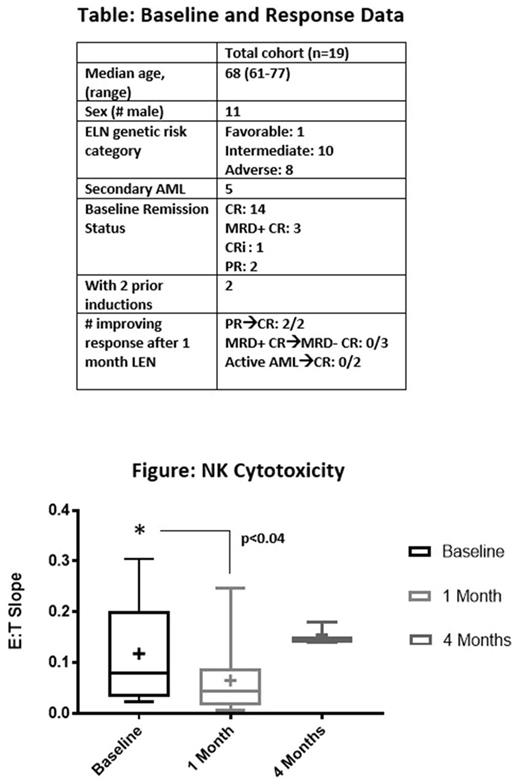
Results: 19 subjects were treated with at least one 28-day course of LEN. Baseline variables are shown in the table. DLTs occurred in 2/9 subjects (hemolytic anemia, rash) at 25 mg, 0/7 subjects at 35 mg, and 1/3 subjects (elevated bilirubin) at 50 mg. No subject in complete remission required hospital admission for adverse events. Among 7 subjects who began LEN with measureable AML, two experienced improvement in AML response category (table). Median relapse-free survival for subjects starting in or entering CR was 4.3 months (range: 1.9-26.9). There was no change in proportion of T- and NK-cell subsets before and after 1 month of LEN. However, a significant decline in NK cell cytotoxicity was observed after month 1, compared with baseline cytotoxicity (p=0.04, paired t test, figure). Among three subjects with NK cell cytotoxicity measured after 4 months of LEN, there was a non-significant trend toward higher cytotoxicity than at baseline and 1-month (figure). Serial geriatric assessments did not demonstrate changes in subject physical, cognitive, psychological or social function.
Conclusions: High dose LEN can be safely administered to older patients with AML after induction. Expansion of the 35 mg dose cohort is ongoing. Unexpected declines in NK-cell cytotoxicity may be due to delayed reconstitution of NK- and T-cell numbers in older patients who have been recently treated with induction therapy. Further investigation should explore responses to LEN in patients with low levels of residual disease, the impact of LEN on geriatric assessment domains, and on the T- and NK-cell repertoire after >1 month of LEN.
Foster: Celator Pharmaceuticals: Research Funding; Pfizer, Inc.: Research Funding; Macrogenics, Inc.: Research Funding; Amgen, Inc.: Honoraria; Shire: Honoraria; Celgene Corporation: Research Funding; Incyte: Honoraria. Vincent: Merck: Research Funding; Pharmacyclics: Research Funding; Nanostring: Membership on an entity's Board of Directors or advisory committees. Zeidner: Tolero Pharmaceuticals: Research Funding; Takeda: Research Funding; Tolero Pharmaceuticals: Honoraria; Celgene: Honoraria; Agios: Honoraria; Merck: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal